Dr. Lee’s research focuses on understanding electrochemical energy conversion and storage mechanisms as well as on designing electrochemical systems that can link renewable energy sources with various end-user applications. The development of these technologies is a critical factor in supporting new transportation technologies, load-leveling for solar and stationary power applications, and fast-evolving portable electronic devices. To respond to these challenges, we will explore the following interconnected research areas.
High-Performance and Multi-Functional Batteries
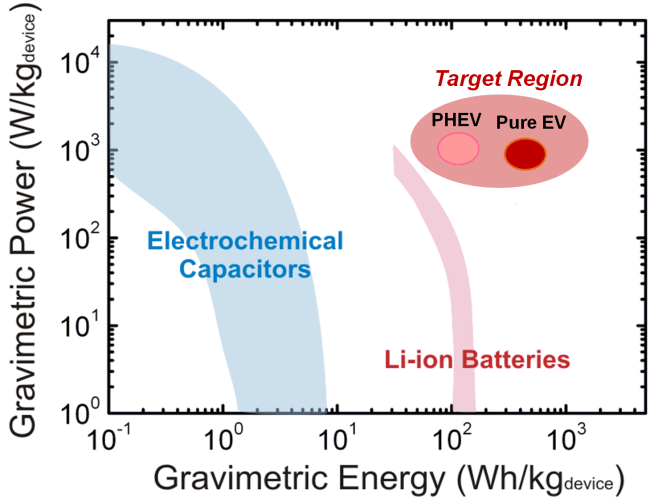
Two major electrochemical energy storage technologies are lithium-ion batteries, which can store high energy of ~150 Wh/kg but have relatively low power (<1kW/kg), and electrochemical capacitors (ECs), which have much higher power capabilities (10 kW/kg) but are limited for low energy densities (<10 Wh/kg) (Scheme 1). However, next-generation energy storage applications, such as load-leveling, flexible electronics, and electrified propulsion, require multi-functional energy sources that have high energy and power, long cycle life, compact size, and flexibility, exceeding the performance of conventional lithium-ion batteries and ECs.
To meet these demands, we will focus on developing novel energy storage devices having multi-functionality as well as high performance. We will first design novel redox couple-nanocarbon batteries to reach the performance target region for electric vehicle applications (Scheme 1).To find novel redox couples having high energy density, we will further explore the energy storage mechanism and reaction kinetics of nanoscale redox couples having multiple oxidation states in relation to their size as well as surface and electronic structure, and synthesize these materials with high redox potential and facile charge transfer. On top of these fundamental studies, we will first increase the capacity of the electrodes by increasing the number of electrochemically accessible functional groups per unit mass or volume of carbons. Next, we will design finely tuned specific functional groups with high redox potentials, and will incorporate these molecules into the conductive carbon matrix to provide both high energy and high power performance with cycling stability.
Electrocatalytic Solar-to-Fuel Energy Conversion
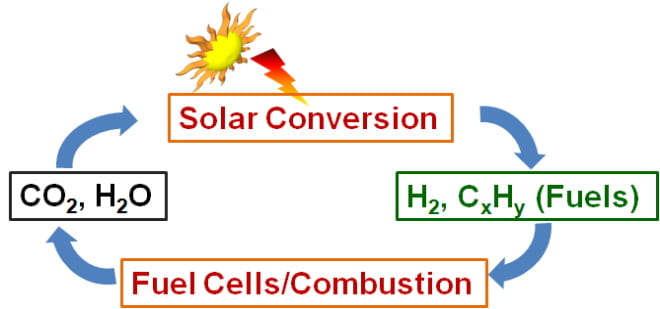
Electrocatalytic solar-to-fuel energy conversion is an attractive way to store solar energy in chemical bonds of fuels. These reactions (Scheme 2) begin with solar energy conversion in the photovoltaic materials, generating holes and electrons. These holes drive water-splitting (oxidation) reaction at the anode. Simultaneously, the photo-generated electrons drive CO2 reduction reaction in water at the cathode, resulting in the production of hydrocarbon and hydrogen fuels. Then, generated fuels are transformed to energy via fuel cells or combustion engines and are converted back to water and CO2, closing the solar-to-fuel energy conversion cycle in a carbon-neutral way. However, the development of this system requires efficient electrocatalysts, which can reduce high overpotential and have selectivity to useful fuels in fuel-forming reactions. A fundamental understanding of the structure and function of the catalytic active site can provide guidance in designing more active, selective and stable catalysts. To understand key design factors, we will study the electrochemical reaction activity, selectivity, and durability of electrocatalysts in correlation with the geometric and electronic structure of catalyst surfaces. From these findings, we will synthesize highly efficient CO2 reduction and water-splitting catalysts from earth-abundant transition metal oxides. Finally, these engineered electrocatalysts for CO2 reduction and water-splitting reactions will be integrated on semiconductor photovoltaic materials to fabricate a photo-electrocatalytic reactor.